PT-141
RESEARCH USE ONLY
These compounds are NOT intended for human consumption, clinical use, or veterinary applications. We are not affiliated with any pharmaceutical companies or their commercial medications. By placing an order, you certify these materials will be used exclusively for in vitro testing and laboratory experimentation only. Bodily introduction of any kind into humans or animals is strictly forbidden by law. This product should only be handled by licensed, qualified professionals. This product is not a drug, food, or cosmetic and may not be misbranded, misused or mislabeled as a drug, food or cosmetic.

| Product Specifications | |
|---|---|
| Application | |
| Appearance | |
| Chemical Formula | |
| PubChem CID | |
| CAS Number | |
| Molecular Weight | |
| Synonyms | |
| PT-141 10mg Storage |
Related Peptides
You ask,
we answer.
Are these peptides quality tested?
Are these peptides quality tested?
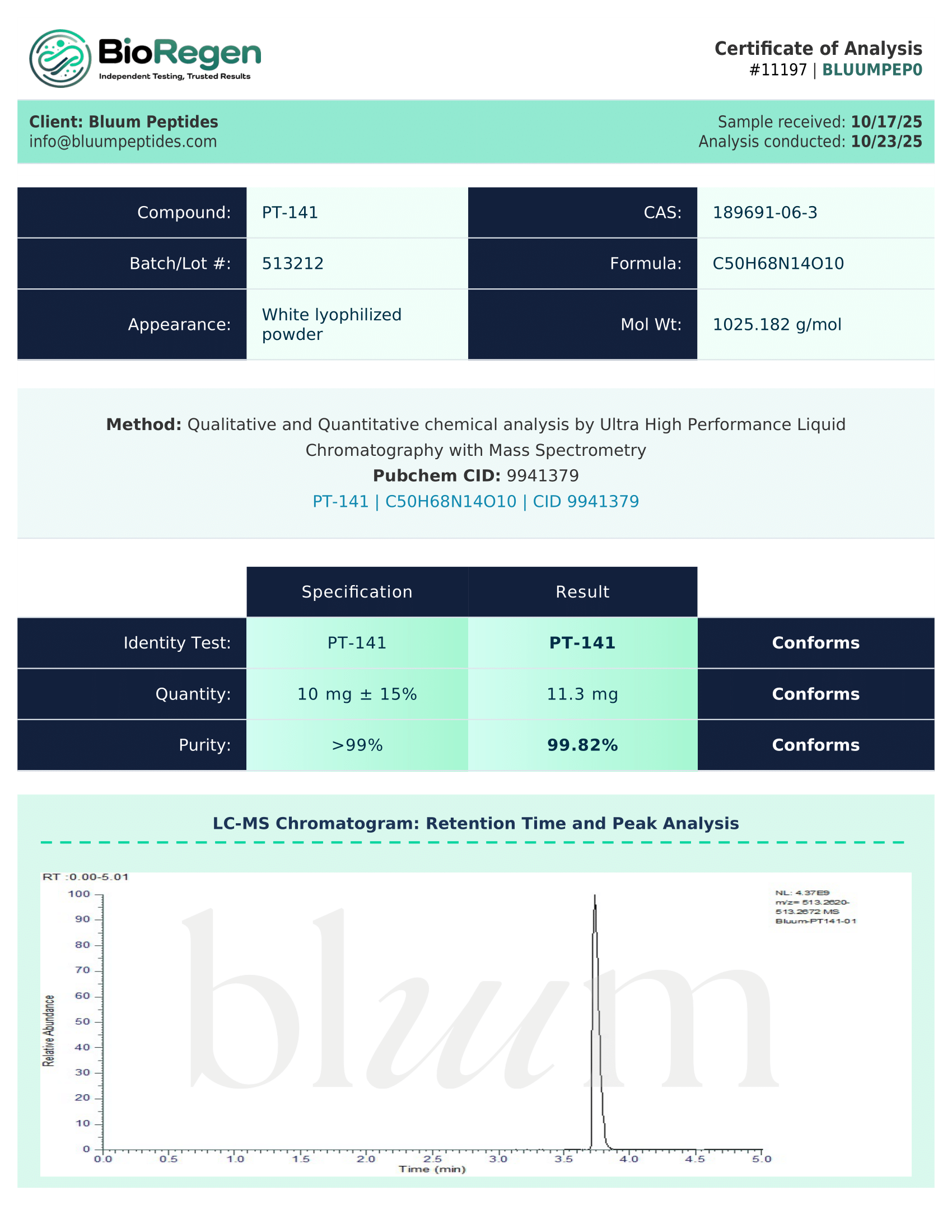
Absolutely. Our analytical testing is conducted by Janoshik Analytical or BioRegen, independent third-party laboratories to verify the identity, purity, and composition of our research products. Each CoA includes purity analysis, peptide sequence confirmation, and date of analysis.
What are typical delivery times?
What are typical delivery times?
Delivery for our free shipping option typically takes 3-5 business days. We ship from right here in the USA to all US addresses. We also provide an option for next day shipping for an extra charge. Please allow 24 hours for processing.
Every package comes with professional packaging, tracking updates via email, and does not require a signature upon delivery.
How should these compounds be stored?
How should these compounds be stored?
Our peptides are shipping in lyophilized form, which is stable at room temperature during transit. Once received, store unopened vials in a cool, dry place.
Are products stable during shipping?
Are products stable during shipping?
Our peptides are shipped in lyophilized (freeze-dried) form, which ensures stability during transit. This powder form is highly stable at room temperature and resistant to temperature fluctuations that occur during shipping.
Research has shown no significant degradation or loss of purity when lyophilized peptides are exposed to room temperature during typical shipping timeframes. Each batch is verified for purity upon production, and our stability testing confirms maintenance of product integrity during standard shipping conditions.
What are your bulk ordering options?
What are your bulk ordering options?
For bulk inquiries and volume pricing, please contact us.
Are these peptides legal to buy for research in the USA?
Are these peptides legal to buy for research in the USA?
Yes. When purchased for laboratory/research use only and handled in compliance with all applicable federal, state, and local regulations. We sell reagents labeled “Not for human consumption,” not as drugs or supplements.
Some compounds may be restricted in certain jurisdictions. The buyer is responsible for knowing and following their local rules.
What is the shelf life/expiration of unopened vials?
What is the shelf life/expiration of unopened vials?
Each lot lists a best-by/expiration on the vial label and COA. As general guidance, lyophilized peptides stored as directed are typically stable 12–24 months (often longer at –20 °C). Short shipping periods at ambient temperature are normal. Actual stability depends on sequence and storage conditions.